

Although nuts are energy-dense foods, they do not predispose to obesity, and in fact may even help in weight loss. Nut consumption also confers modest improvements on glycemic control, blood pressure (BP), endothelial function, and inflammation. Randomized controlled trials consistently show nuts have a cholesterol-lowering effect. Favorable effects on cognitive function and depression have also been reported. Nut consumption is correlated with lower cancer incidence and cancer mortality, and decreased all-cause mortality. There is increasing evidence that nuts positively impact myriad other health outcomes as well. These nut components synergize to favorably influence metabolic and vascular physiology pathways, ameliorate cardiovascular risk factors and improve cardiovascular prognosis.
Nutrfs trial#
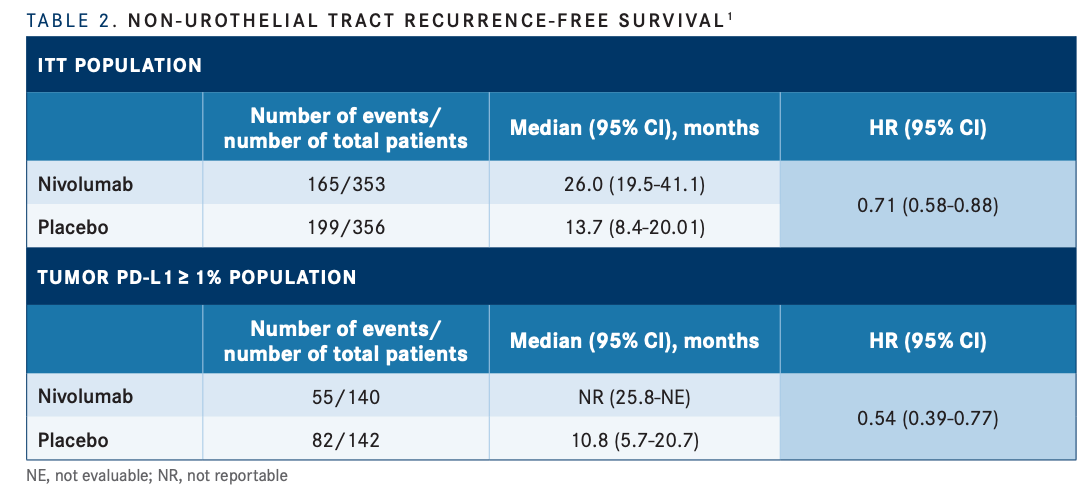
Clinical trial information: NCT02632409.Common nuts (tree nuts and peanuts) are energy-dense foods that nature has gifted with a complex matrix of beneficial nutrients and bioactives, including monounsaturated and polyunsaturated fatty acids, high-quality protein, fiber, non-sodium minerals, tocopherols, phytosterols, and antioxidant phenolics. These results further support adjuvant NIVO as a standard-of-care treatment for pts with high-risk MIBC after radical resection ± neoadjuvant cisplatin-based chemotherapy. The DFS benefit was observed in all prespecified subgroups. Improvement in DFS was observed with NIVO over PBO in pts with MIBC after radical resection regardless of tumor PD-L1 expression. Grade 3–4 treatment-related adverse events occurred in 17% and 6% of pts in the NIVO and PBO arms, respectively. Improvement in NUTRFS and DMFS with NIVO vs PBO was also observed (Table). DFS was improved with NIVO vs PBO across subgroups according to age, sex, ECOG performance status, nodal status, and PD-L1 expression status. DFS probability at 12 months in all MIBC pts was 66% with NIVO and 45% with PBO. With a minimum follow-up of 11.0 months, a DFS benefit was observed with NIVO vs PBO in these pts, regardless of tumor PD-L1 expression (Table).

Of 709 randomized pts in the trial, 560 had MIBC (NIVO, n = 279 PBO, n = 281). This exploratory analysis focused on the subgroup of pts with muscle-invasive bladder cancer (MIBC) after radical resection. Non–urothelial tract recurrence-free survival (NUTRFS) was a secondary endpoint, and distant metastasis-free survival (DMFS) was an exploratory endpoint. Primary endpoints were DFS in ITT pts and in pts with PD-L1 ≥ 1%. Pts had radical resection ± neoadjuvant chemotherapy and were at high risk of recurrence on final pathologic staging. Pts were randomized 1:1 to NIVO 240 mg intravenously every 2 weeks or PBO for ≤ 1 year of adjuvant treatment and stratified by nodal status, prior neoadjuvant cisplatin, and tumor PD-L1 expression. We report results for the subgroup of pts with bladder cancer, the most predominant type of urothelial carcinoma.ĬheckMate 274 is a phase 3, randomized, double-blind trial of adjuvant NIVO vs PBO in high-risk muscle-invasive urothelial carcinoma (bladder, ureter, renal pelvis) after radical resection. In the CheckMate 274 trial, disease-free survival (DFS) was significantly improved with nivolumab (NIVO) vs placebo (PBO) both in intent-to-treat (ITT) patients (pts) (hazard ratio, 0.70 98.22% confidence interval, 0.55–0.90 P < 0.001) and in pts with tumor programmed death ligand 1 (PD-L1) expression ≥ 1% (HR, 0.55 98.72% CI, 0.35–0.85 P < 0.001).


 0 kommentar(er)
0 kommentar(er)
